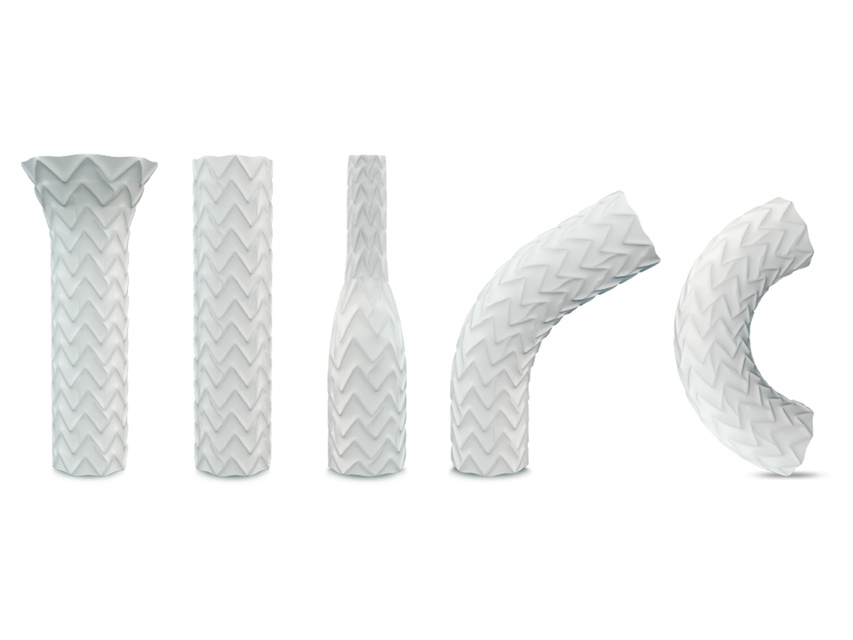
Advanta V12 balloon expandable covered stent
Advanta V12 is the first to market balloon expandable, fully encapsulated stent. Known for its precision and predictability – the versatile Advanta V12 covered stent has been meeting the needs of surgeons and patients for 20 years, and is the only durable solution backed by decades of real-world evidence.[1][2][3]
The Advanta V12 covered stent system indications:[2]
- Treatment of patients diagnosed with renal artery stenosis and/or aortoiliac occlusive disease, including lesions at the aortic bifurcation, when endovascular therapy is required.
- Treatment of patients with aneurysmal disease, when fenestrated endovascular aortic repair (FEVAR) or branched endovascular aortic repair (BEVAR) is required.
- Treatment of patients with iliac artery aneurysmal disease, requiring treatment with an iliac branch device (IBD).
The Advanta V12 balloon expandable covered stent is not available in the U.S.

Advanta V12
Durability
isn't optional. It's vital.
Choose the stent that's designed – and proven – to go to the distance, giving you confidence with every crossing.[2]

Predictable & Precise
- Low profile, reliable stent retention, and secure trackability facilitate stent implantation [2]
- 6 and 7 French compatible on 5-10 mm sizes. 6 French compatible with most
common renal sizes. [2] - Predictable recoil and foreshortening promotes precise deployment [2]
- Full encapsulation with ePTFE helps mitigate the risks related to vessel perforation [2][6][9][13]
- Radiopaque markers enhance visibility during deployment and assist with accurate stent placement [2]
- Dog‐bone inflation design is intended to reduce the chances of embolization [7]

Versatile & Flexible
- Stent structure, cell design, and system provide versatility and flexibility in delivery and placement [2]
- Designed for pushability and trackability through tortuous anatomy with conformance to iliac, renal, superior mesenteric, and celiac arteries.[2]
- Able to post-dilate and flare stent, conforming to the anatomy and customizing each patient's treatment [1][2][8]
- Smooth inner lumen offers ease of navigation during re-intervention [2]
* Please see the Advanta V12 order information found in the documents tab for detailed compatibility.
Refer to Instructions for Use for current indications, warnings, contraindications and precautions.
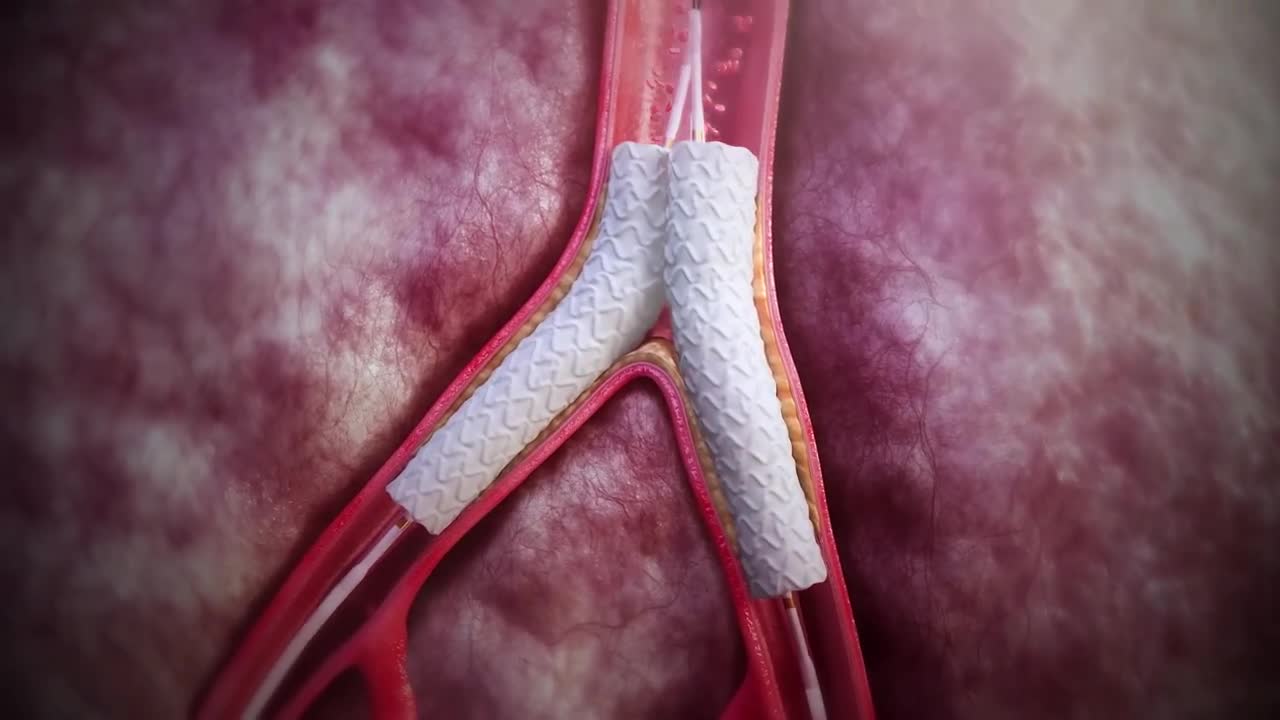
Kiss with confidence [1][2][3][5][7][12]
Advanta V12 is EU MDR approved to treat occlusive lesions at the aortic bifurcation in a kissing configuration.[2]
Evidence spanning over 15 years has shown that using the kissing stent technique, at the aortic bifurcation, treats complex aortoiliac lesions, resulting in technical success, symptom improvement, and sustained patency.[2][5][7][12]
Optimized patient outcomes today, tomorrow and into the future. [1][2][3][4][5][10][11]
- Published literature over the last 20 years supports safety and performance[1][3][4][5][10]
- Proven two-fold lower reintervention compared to bare metal stents at 5 years post-procedure[1]
- Full encapsulation with ePTFE minimizes neointimal hyperplasia formation[2][11]
- 316L stainless steel struts provide additional radial force, designed to support the stent patency[2]
COBEST - 5 year results: Advanta V12 vs. Bare Metal Stent [1]
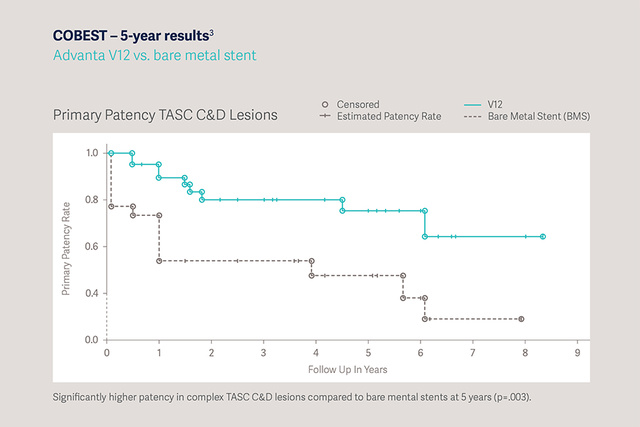
- Significantly higher patency in complex TASC C&D lesions compared to bare metal stents at 5 years (p=0.003).
Systematic review of covered balloon-expandable stents for treating aortoiliac occlusive disease [3]
- The Advanta V12/iCast balloon expandable covered stent has long-term, real-world follow-up, including a reported 5-year primary patency rate of 74.7%.
- The Advanta V12/iCast was the most common device studied in the literature from 2000-2019 (10/15 publications; 66.7%).
- The Advanta V12/iCast studies treated patients with more severe disease (a greater number of TASC C&D lesions) and more severe symptoms (more Rutherford classification 4 & 5) compared to patients enrolled in clinical trials studying other covered balloon expandable stents.
- Freedom from TLR: Results were comparable for all covered balloon expandable stents at 1 year. The Advanta V12/iCast has long-term target lesion revascularization data.

iCARUS: Single-Arm IDE study with 3-year follow-up [4]
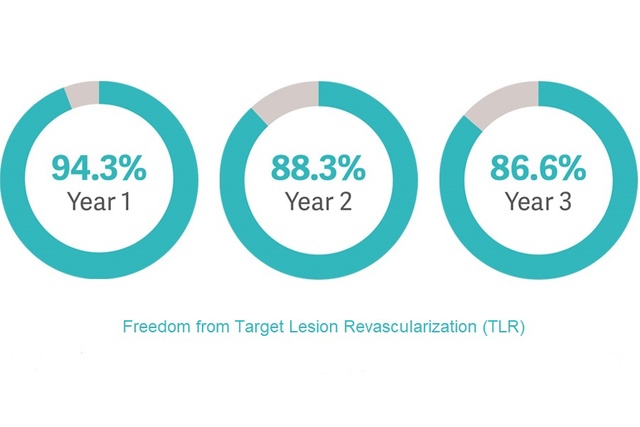
- Real-world patient population with multiple lesions and bilateral disease.
- Study showed sustained clinical benefit with freedom from Target Lesion Revascularization (TLR) up to 3 years.
Kissing Stent deployment for aortoiliac occlusive disease with Advanta V12
Bilateral iliac artery occlusion
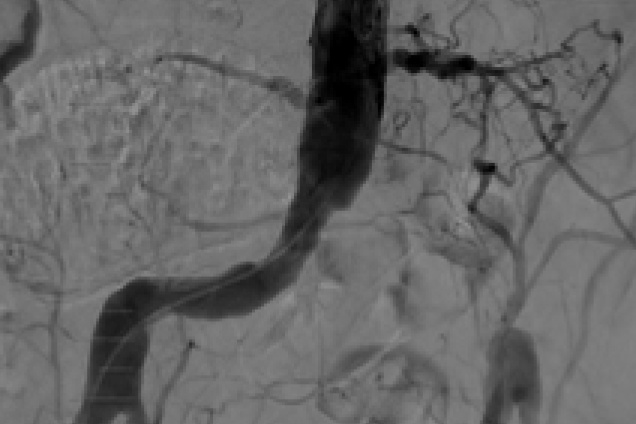
Pre treatment
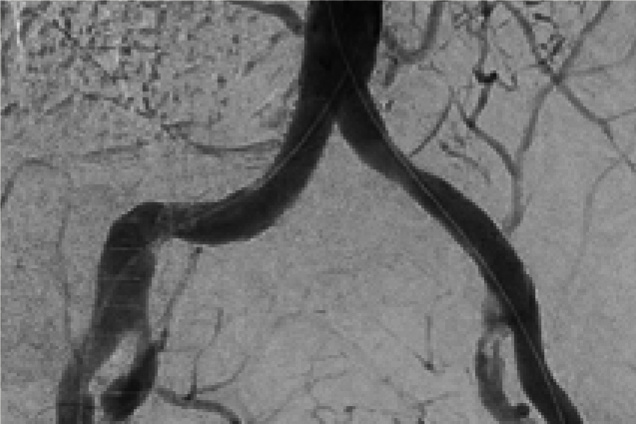
Post treatment
Restoration of lumen diameter with Advanta V12 Kissing Stents. 10 x 38 mm Advanta V12 in the RIA; 10 x 59 mm Advanta V12 stents overlapped in LIA.
RIA - Right Iliac Artery, LIA - Left Iliac Artery
Bilateral common iliac artery occlusion
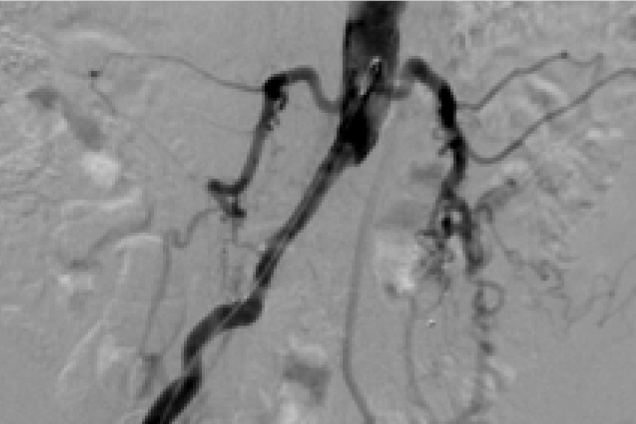
Pre treatment
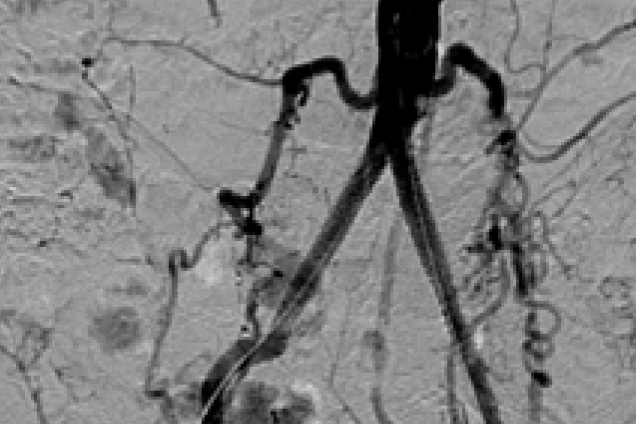
Post treatment
Restoration of the lumen diameter with 8x59 mm Advanta V12 Kissing Stents in RIA and LIA.
RIA - Right Iliac Artery, LIA - Left Iliac Artery
Treatment of renal artery occlusive disease with Advanta V12
Renal artery stenosis
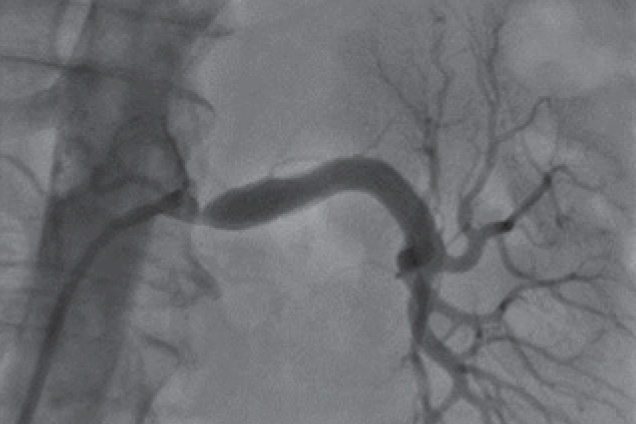
Pre treatment
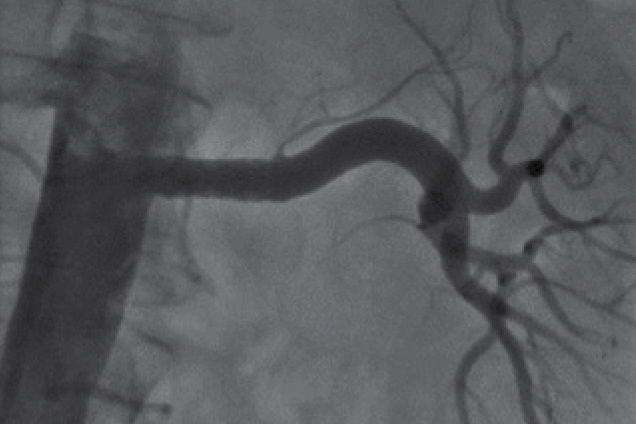
Post treatment
Restoration of the lumen diameter with Advanta V12 covered stents in LRA.
LRA - Left Renal Artery