Secure sterile transfer in pharmaceutical production
The DPTE® system is the industry benchmark for secure sterile transfer in pharmaceutical production. It reduces manual intervention and so lowers contamination risk.
Sterile transfer of components and other materials from one sterile area to another has always been a major concern in the pharmaceutical production area. The ingenious patented DPTE® system provides a leaktight, bi-directional solution.
Safe and secure transfer
The DPTE® system was originally designed as a solution for safe and secure transfer of nuclear products. It’s now the norm in biopharma production with more than 40,000 units installed worldwide.
Getinge's White Paper explains how the DPTE® Alpha and Beta parts combine to securely connect two sterile volumes and move them through a less sterile zone, in order to transfer aseptic or toxic materials with no leakage.
Particulate and microbial leak tests were carried out under rigorously controlled test conditions to demonstrate the performances of DPTE® containers in maintaining sterility.
This validatable and reproducible performance in safety, airtightness and protection against particulate and microbial contamination sets the DPTE® system at the top in the industry.
How does the DPTE® system work?

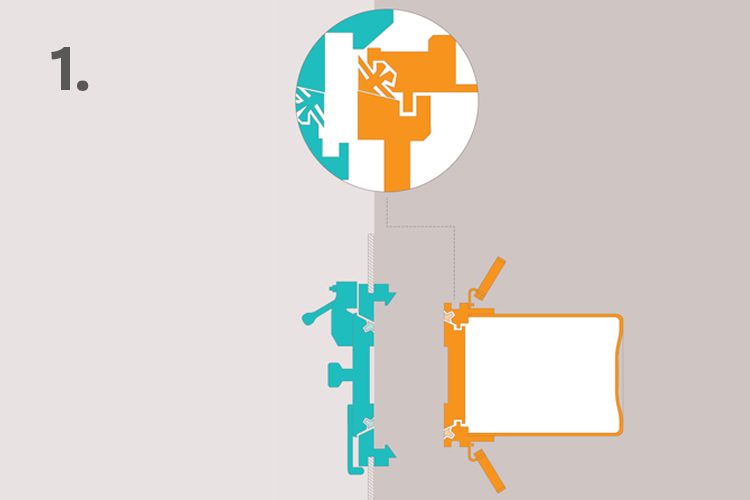
1. Line up the DPTE® Alpha and Beta parts
The Alpha part is mounted on a support – commonly an isolator, RABS, BSC or cleanroom – while the Beta part consists of a container, bags or similar device used for the transfer of components, solids or liquids.
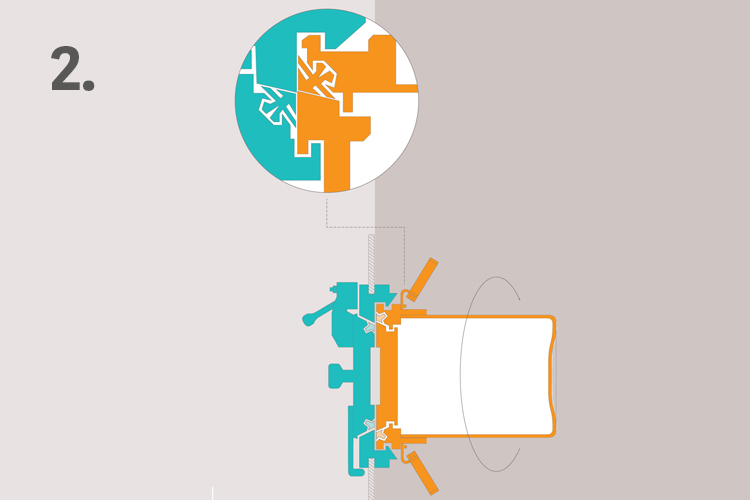
2. Rotate the Beta part 60° to ensure leak tight seal
The Alpha parts and Beta parts are connected by a manual 60° rotation which detached the doors from their supports and joins them together. Tightness is secured by the lip seals of the new assembly.
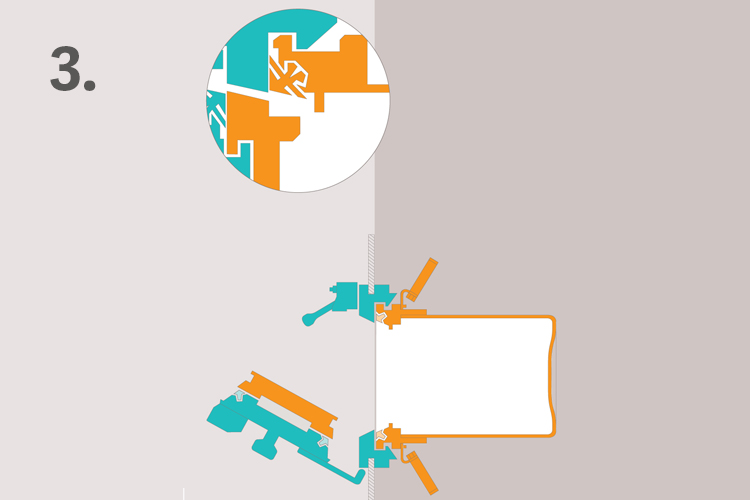
3. Open the doors without breaking sterility or containment
The doors can now be opened without breaking sterility or containment. The combination of DPTE® Alpha and DPTE® Beta parts is a validated solution. Used together, they provide highly secure transfer and protect your production.
Secure sterile transfer in pharmaceutical production

The DPTE® system is the industry benchmark for secure sterile transfer in pharmaceutical production. It reduces manual intervention and so lowers contamination risk.
Learn more about a tried and tested method to minimize microbial contamination while keeping up the pace in production.
