Insights from implementation in the ICU and daily clinical use
Physician and researcher Dr. Heder de Vries, MD, PhD candidate shares his insights into the implementation of Esophageal and Transpulmonary pressure monitoring, and how recent improvements in Pes & PL monitoring are contributing to better support its daily clinical use.
Some of the topics that Dr. de Vries discusses include: The usability of esophageal balloon catheters with feeding tubes. Patient categories where a Pes balloon catheter is being used. Clinical triggers that indicate placement of a Pes balloon catheter. The length of time after ICU admission that a Pes catheter is used. The target range for PL being considered during controlled mechanical ventilation. The key factors when implementing TP in a multi-disciplinary ICU team. The most important research questions regarding the use of Transpulmonary pressure in the next years.
Watch the full video interview below
Learn how to start monitoring Esophageal and Transpulmonary pressure with the Servo-u ventilator system
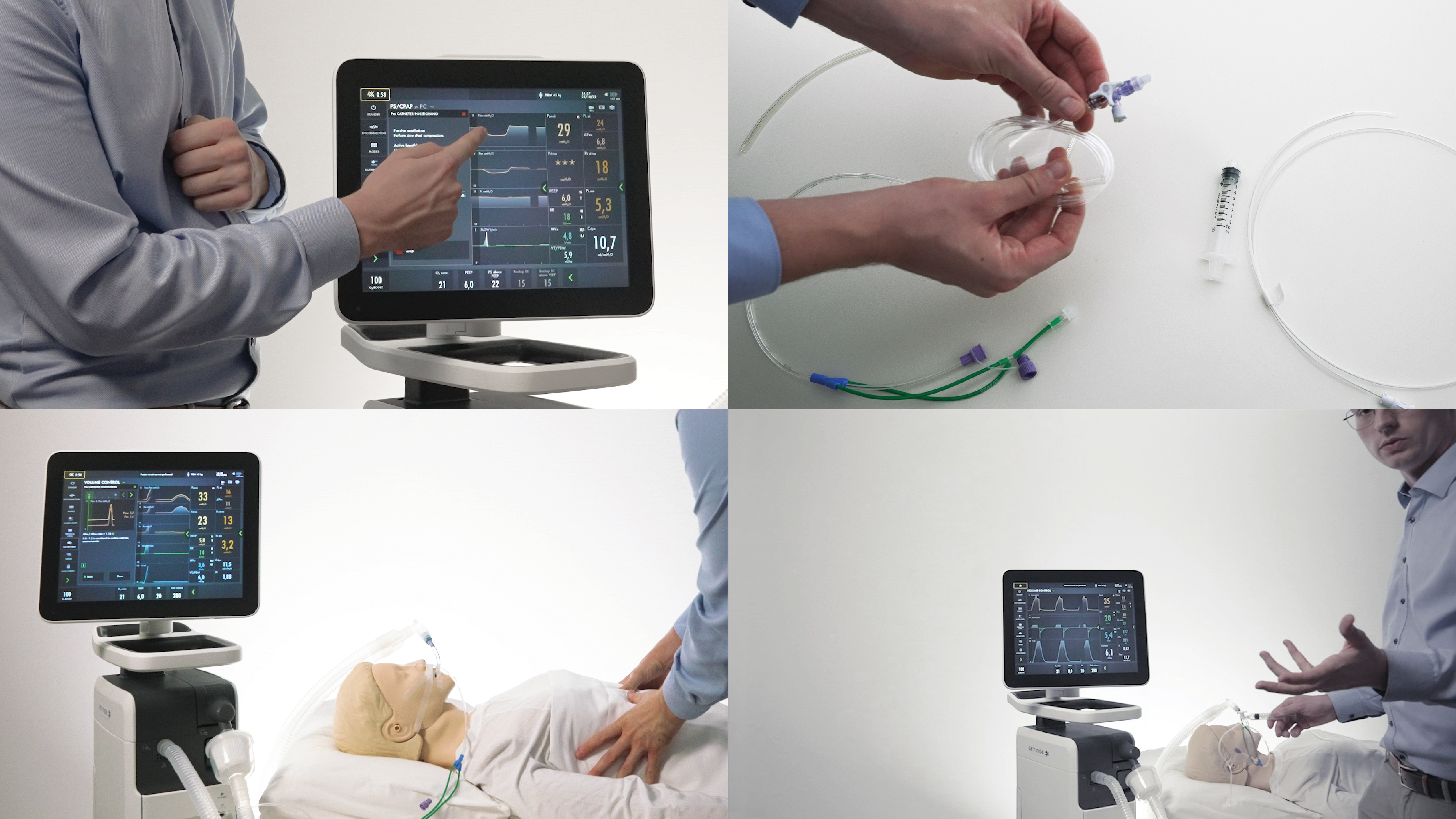
Learn how to start monitoring Esophageal and Transpulmonary pressure with the Servo-u ventilator system. A step by step instructional training video by Dr. Heder de Vries in how to implement Transpulmonary pressure monitoring in a multi-disciplinary ICU team.
Items required for upgrading your Servo-u with Esophageal and Transpulmonary pressure monitoring
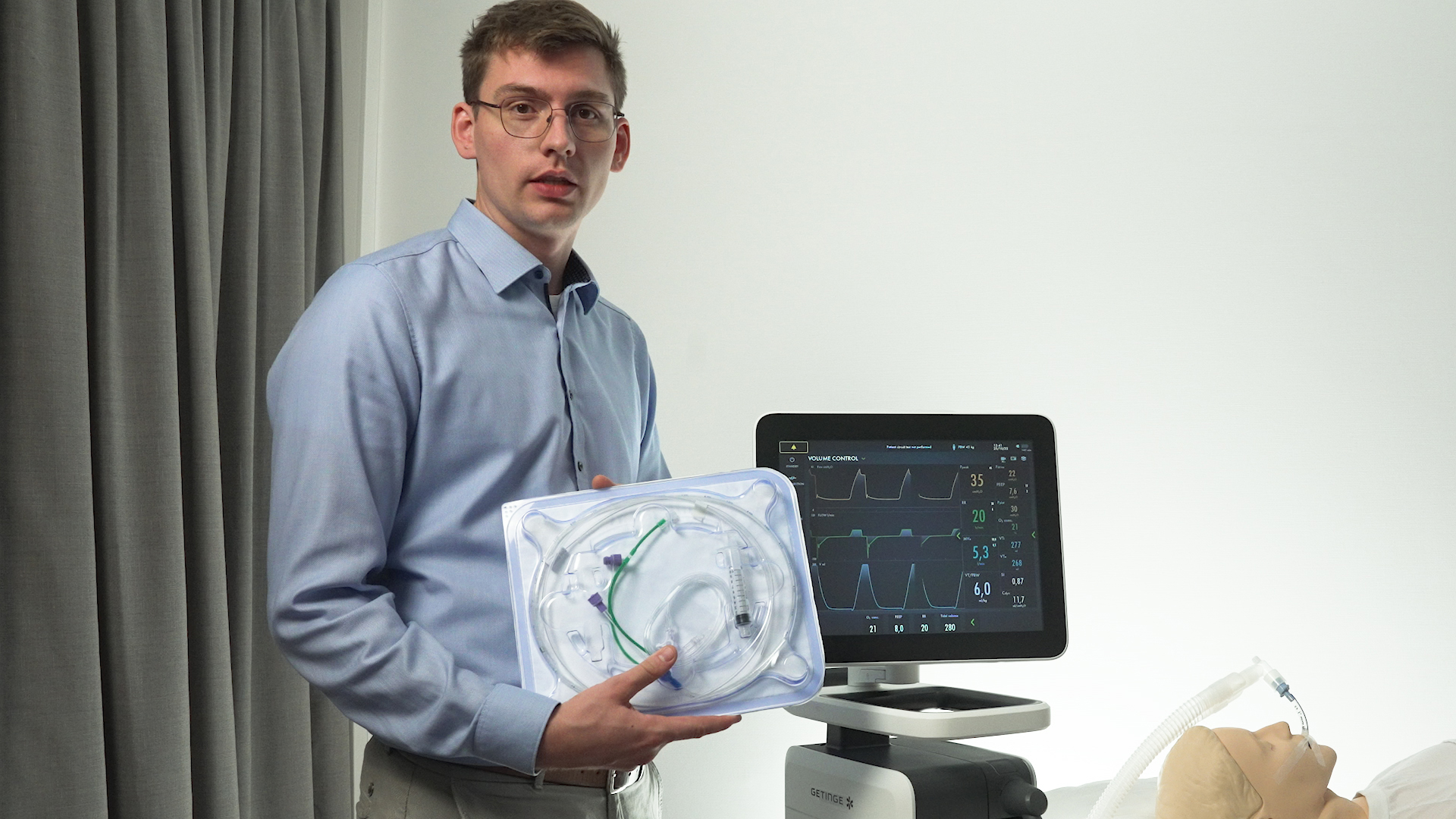
Expand your Servo-u mechanical ventilator systems personalized lung protection therapies with the Transpulmonary pressure monitoring tool. The flyer below describes the items needed for implementation.

