How diaphragm monitoring can help you improve mechanical ventilation
Respiratory support is a lifesaving intervention in the ICU, but without the right balance it also increases the risk of detrimental outcomes.[1],[2] This is when diaphragm monitoring can help, because it is a marker of outcomes such as hospital mortality and prolonged weaning. Furthermore, it can help you make more informed therapy decisions throughout respiratory treatment.
The clinical impact of diaphragm injury
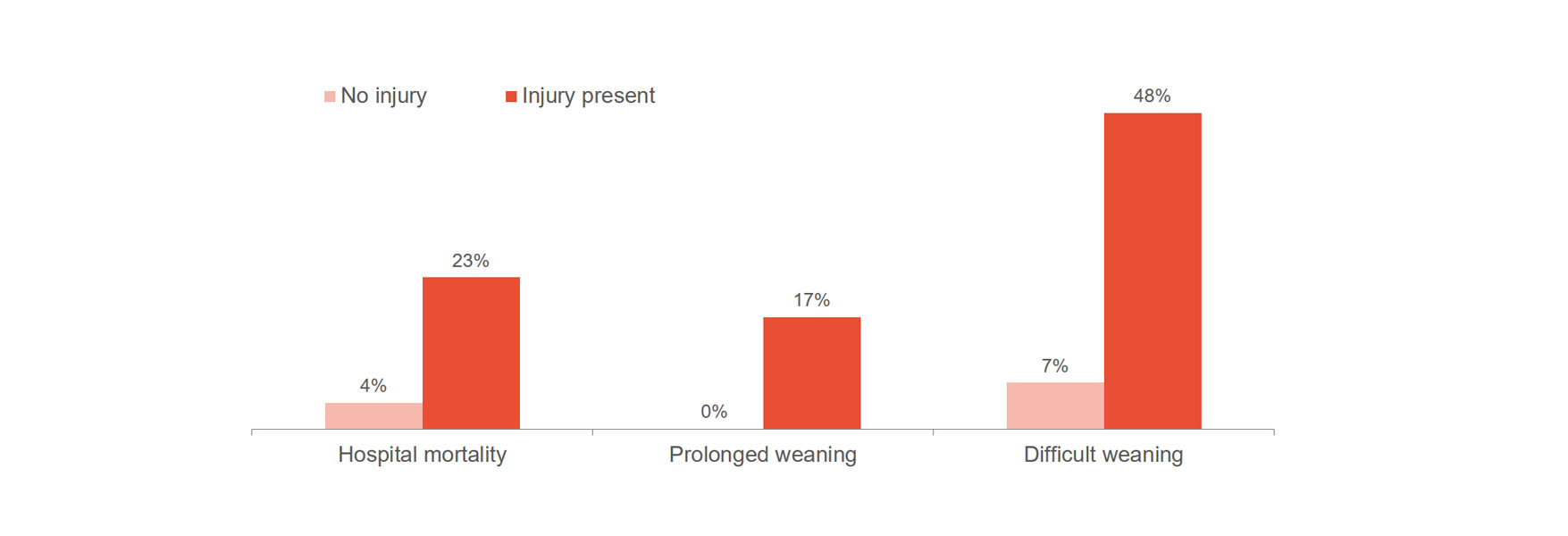
Diaphragm injury significantly increases the risk of worsened outcomes, such as difficult weaning, prolonged weaning and hospital mortality.[1],[2] Importantly, 23-84% of patients exhibit significant diaphragm injury at the first spontaneous breathing trial.[3] One study showed an average time on ventilation of 576 hours for patients with diaphragm injury, compared to 203 hours for patients without injury.[4]
Main causes are believed to be driven by two factors.[1] In some cases, patients are working too hard to breathe, leading to thickening of the diaphragm. In other cases, patients are working too little subsequent to over-assist and/or controlled ventilation early in their treatment, leading to atrophy. Both changes worsened outcomes and the challenge for clinicians today is that commonly used ventilator diagnostics fail to capture this information.
Why current ventilator diagnostics are not enough
Ventilator curves are used to interpret patient respiratory needs, but their primary function is to show what you deliver to the patient. This makes it difficult to detect asynchrony, over-sedation, over-assist and under-assist during spontaneous breathing.
For example, only 21% of clinicians detect asynchrony in the form of missed inspiratory efforts.[5] Moreover, a patient on pressure support ventilation can appear to be triggering spontaneous breaths, when in reality they are not triggering any spontaneous breaths at all.[5], [6]
The result is uncertainty about how much breathing effort your patient is exerting and to what extent he or she is at risk of diaphragm injury.
How to monitor the diaphragm
In order to try and safeguard the diaphragm, you need to diagnose the potential injury and monitor its continuous activity.
An ultrasound helps you assess diaphragm dysfunction by measuring its thickness and potential changes to thickness over time. Recent advances in ultrasound imaging enable clinicians to more feasibly assess diaphragm function and potentially protect the diaphragm during mechanical ventilation.[7]
For continuous, breath by breath, monitoring of diaphragm function, there is the Electrical activity of the diaphragm (Edi). It is a bedside diagnostic tool obtained through a specially designed feeding tube. The voltage signal is displayed as a waveform alongside the patient's conventional pressure / flow curves, and shows the presence, absence and form of breathing.
Edi can help you understand the work of breathing, detect asynchronies and assess the extent to which over-assist or under-assist and sedation are affecting breathing ability.[8],[9] You can also detect changes in effort following interventions. Examples are when you change the patient's position, administer medications such as Salbutamol or, crucially, when you reduce ventilatory support during weaning.
Arguably, combining ultrasound and continuous diaphragm monitoring (Edi) is needed for the complete picture.
How diaphragm monitoring may help protect the patient and simplify weaning
To avoid ventilator induced lung injury you want to try to avoid invasive ventilation, asynchronies, and over- and under assist and longer periods of sedation and diaphragm inactivity . Patients ‘fighting the ventilator’ often lose. Increased sedation, prolonged ventilation and possibly intubation tend to be the result.
Diaphragm monitoring may help manage these challenges.[10],[11],[12] It helps you see efforts made by the patient, breath by breath. And you can see if the ventilator responds in time, with the appropriate amount of support, because you have an objective, physiological value to guide you.
In non-invasive therapy, this can help you adapt the timing and the support of the ventilator, which may reduce the need to intubate. Good patient-ventilator interaction is one of the key factors of successful NIV.[13]
Continuous monitoring can also act as a real time indicator of breathing effort helping you understand when intubation is really necessary. It may even help you optimize the timing of spontaneous breathing trials as well as progress them more successfully, more often.
How diaphragm monitoring may help decrease time on the ventilator
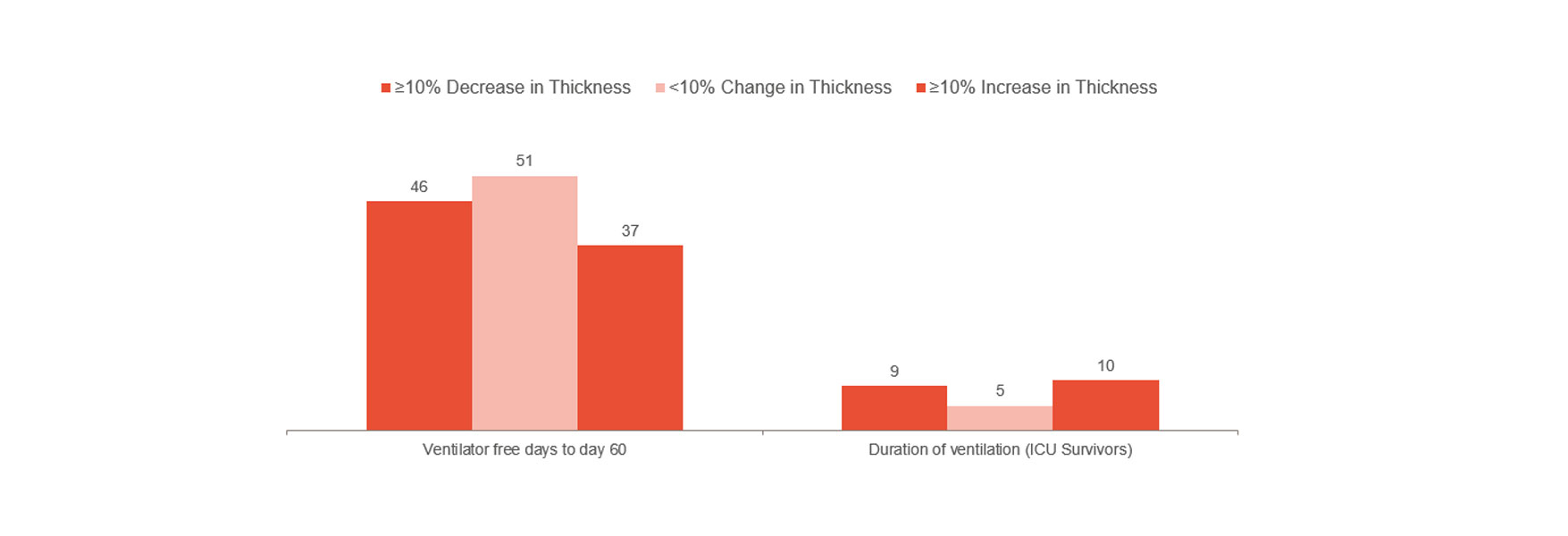
Goligher showed that early change of diaphragm thickness was a marker of ICU length of stay and other complications such as reintubation, tracheostomy, prolonged mechanical ventilation and death.[1] It indicates that staying within a 10-20% thickening fraction may be the optimal way forward. As such, it can give you indications of patient risk and help you optimize treatment. To further understand if the avoidance of diaphragm injury could prevent complications requires randomized clinical trials.
In line with the above, however, is the clinical experience from a London Hospital, which indicated a significant reduction in time spent on mechanical ventilation when monitoring diaphragm activity.[14] The non-monitored group had a median of 12 days on mechanical ventilation compared to a median of 9 days for the monitored group (103 patients of 493).
Monitoring the diaphragm may also help you discover disruptions, such as congenital central hypoventilation syndrome and phrenic nerve damage.[15],[16]
How diaphragm monitoring can help you make more informed treatment decisions
Monitoring diaphragm activity can help you make more informed decisions for your patient throughout treatment and provide valuable information at a number of decision points.
Monitor and trend work of breathing
Recent reports indicate that diaphragm monitoring with Edi is useful to monitor breathing effort and patient-ventilator interaction.[18]
Of course, Edi monitoring has limitations as a single, isolated value. Like other physiological variables, it should be considered in conjunction with other measurements as well as in the context of changes in therapy – a trend over time that can help you determine if your patient is moving in the desired direction.
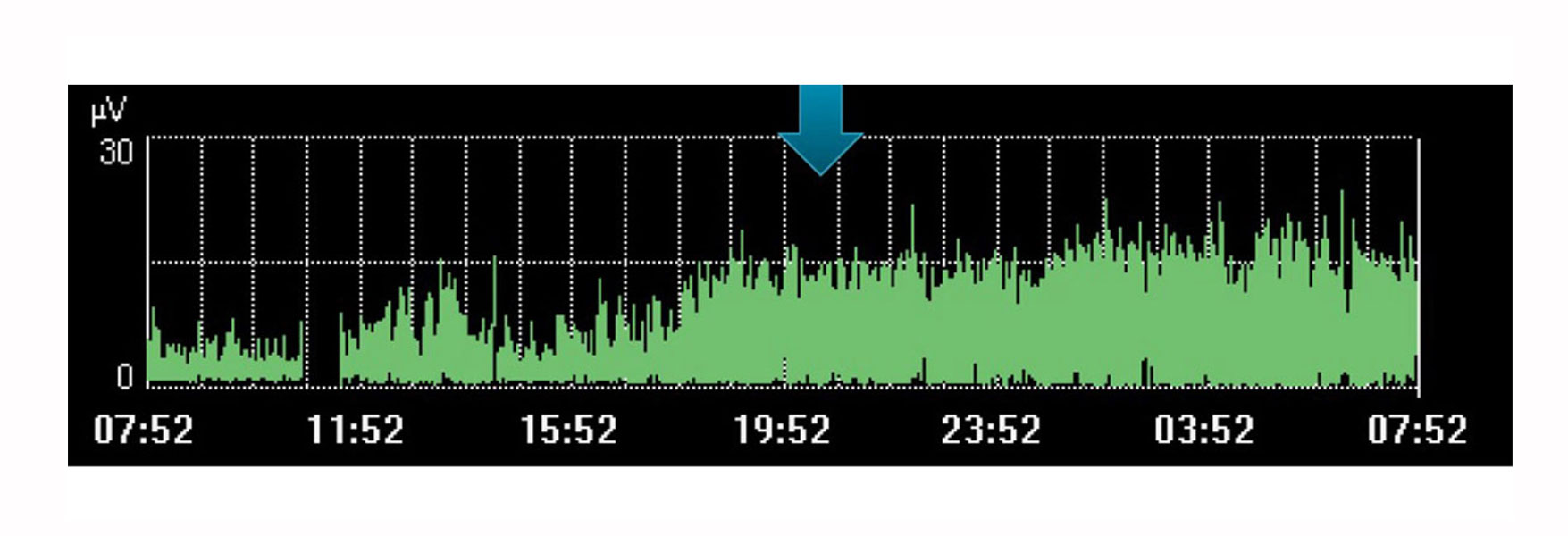
For example, the image above shows an increase of diaphragm effort during a time when the doctor had planned a rest for the patient. The trend indicates it didn’t occur, visible by the increase in effort made by the patient during this time.
Identifying over-assist and under-assist
To keep the patient from diaphragm injury, the diaphragm needs to be active at an appropriate level. This is difficult to see without diaphragm monitoring.
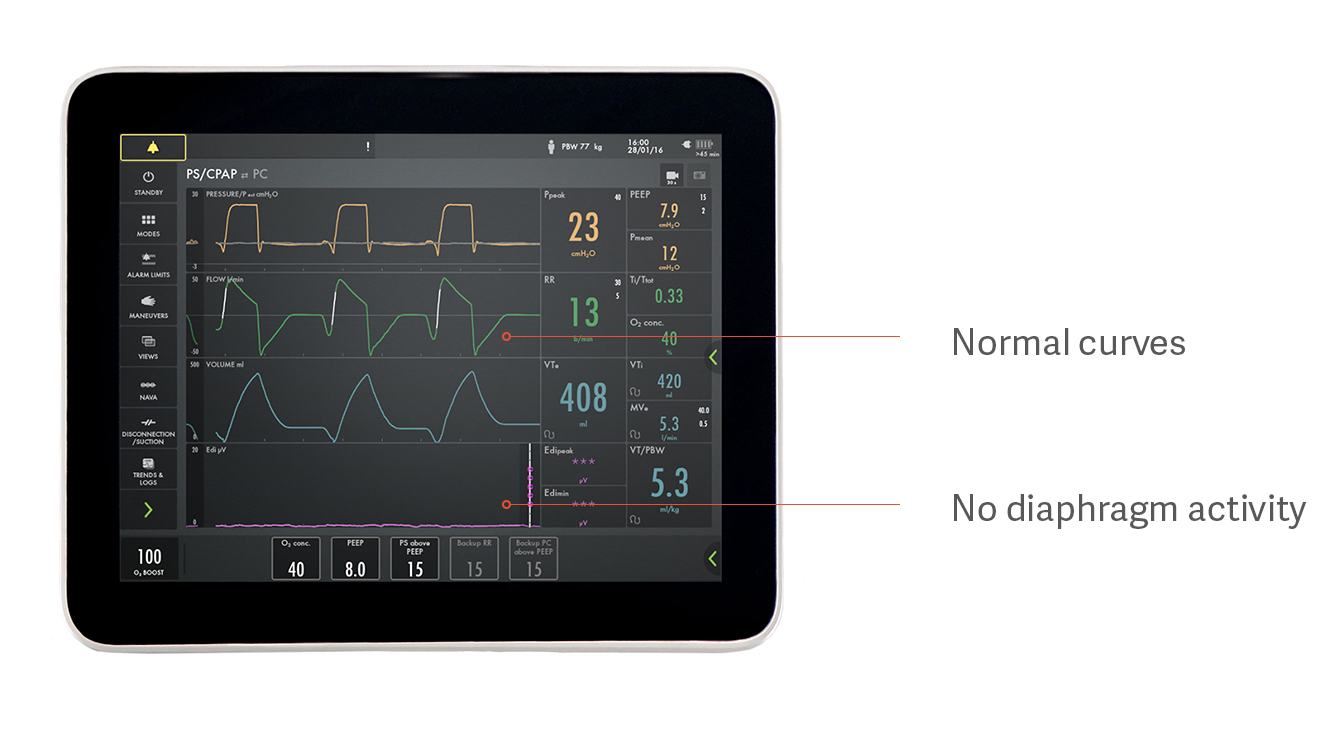
For example, a patient can appear to be spontaneously breathing with pressure support but not, in fact, be using their diaphragm at all, as indicated in the image above. This is one example of how over-assistance prevents the diaphragm from working, resulting in diaphragm atrophy. The pressure, flow and volume curves look normal, but the purple Edi signal at the bottom is flat, indicating an inactive diaphragm.
Another example is under-assist, which is the opposite of over-assist and equally bad for the patient. An under-assisted patient uses too much effort to breathe, resulting in diaphragm thickening. This is perhaps easier to observe in the patient, but without an objective value on the ventilator it is difficult to know for sure.
Both examples of diaphragm injury (atrophy and thickening) are frequently seen in patients and associated with worsened clinical outcomes.[1]
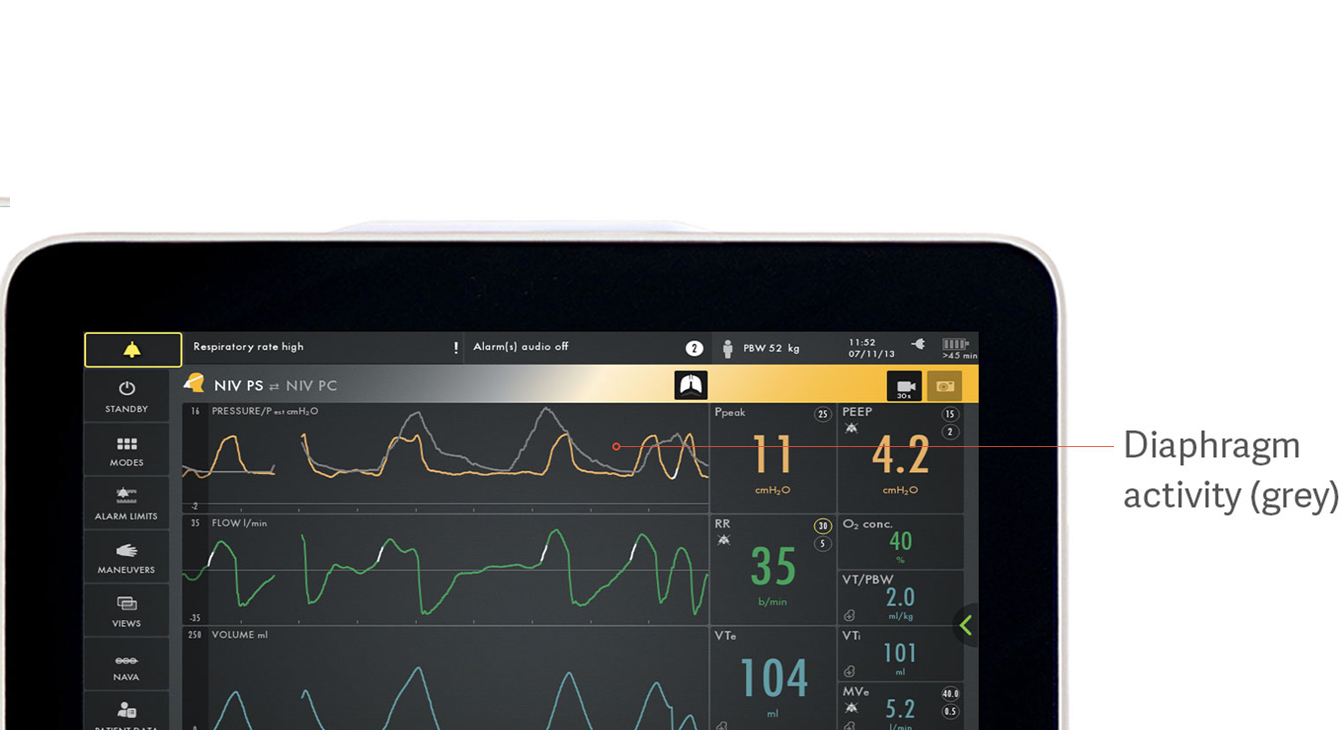
Identifying patient-ventilator asynchrony
Asynchrony is associated with poorer clinical outcomes during mechanical ventilation.[18] In a recent study only 21% of clinicians managed to detect asynchrony in the form of missed inspiratory efforts.[5] There are many more types of asynchronies that are easily overlooked: ineffective or excessive efforts, delayed inspiratory effort, delayed cycling off, double triggering and auto-triggering.
The image shows how the electrical activity of the diaphragm in grey overlays the pressure curve (yellow), making it easy to see differences in what the patient requests and what the ventilator delivers.
Determine mode of ventilation
Your target should be for the patient to sustain an optimal respiratory effort that represents neither too little nor too much effort.[1] By continuously monitoring diaphragm activity, you have an indication of how much the patient is working, if at all. If the diaphragm activity is high and rising, you may have to increase the level of support.[19],[20],[21]
If the activity is low or reducing, you may be able to decrease the level of support.[19] It is important to also monitor other diagnostic parameters associated with ventilation before changing the support. Research is growing in this area. In the future, more knowledge about diaphragm parameters may improve assessment further.[22]
Set an optimal PEEP
There is no standardized way of setting the patient's PEEP during spontaneous breathing. Yet a well set PEEP can decrease atelectasis, cyclical opening and closing of airways and protect alveoli. This, in turn, optimizes lung mechanics and improves oxygenation.
PEEP titration with diaphragm monitoring has shown clear results in neonates, allowing the baby to relax appropriately between breaths and preventing derecruitment of the lungs.[12]
In adult patients, Passath used diaphragm and oxygen monitoring during PEEP changes to allow for identification of a PEEP level at which tidal breathing occurs at minimal effort.[23] Excessive lowering of PEEP resulted in an increase in work of breathing by 50 to 60% which, in combination with worsening of oxygen, also suggested partial lung derecruitment.
Optimizing management of sedation
The main benefit of monitoring diaphragm activity in relationship to sedation is to try and keep the diaphragm active as much as possible.[1] Simply monitor your patient’s diaphragm activity and response to ventilation to find an adequate sedation level with sustained diaphragm activity.
It may require some training to differentiate the effect of the sedation from other physiology that may impact diaphragm function, however, Edi is particularly effective during sedation holds as you can continuously see the changing effort made by the patient.
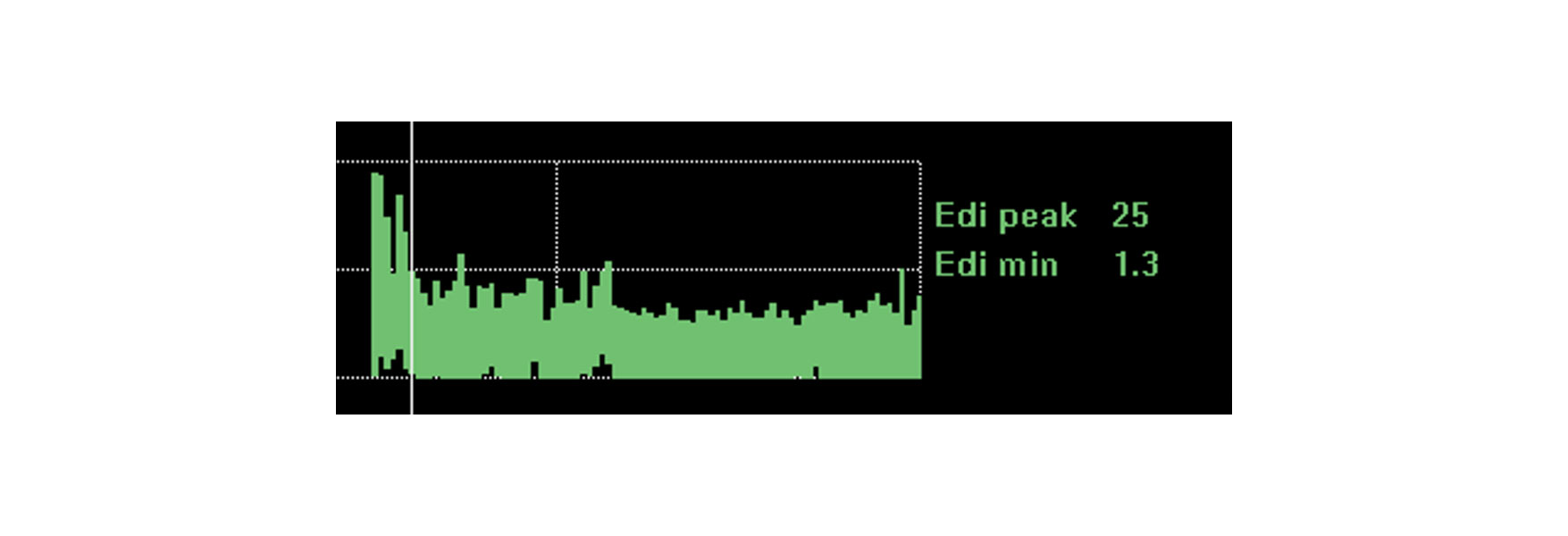
Trend and monitor the impact of interventions, rest and rehabilitation
Monitoring diaphragm activity provides further reassurance that the patient can cope with the changes you make. Diaphragm activity is impacted by a range of physiological changes such as rest, sitting up, walking, caffeine treatment and even global rehabilitation and recovery.
If the patient is coping with these changes, diaphragm activity may remain largely unchanged. A worsening of the clinical situation and a requirement for greater respiratory work will most likely increase the diaphragm activity. An improved resting position will lower diaphragm activity needed to generate breaths.
The image shows the continuous diaphragm activity of a patient who was about to be intubated due to acute respiratory distress following pneumonia. By monitoring diaphragm activity, the clinician managed to optimize the support and turn the situation around.
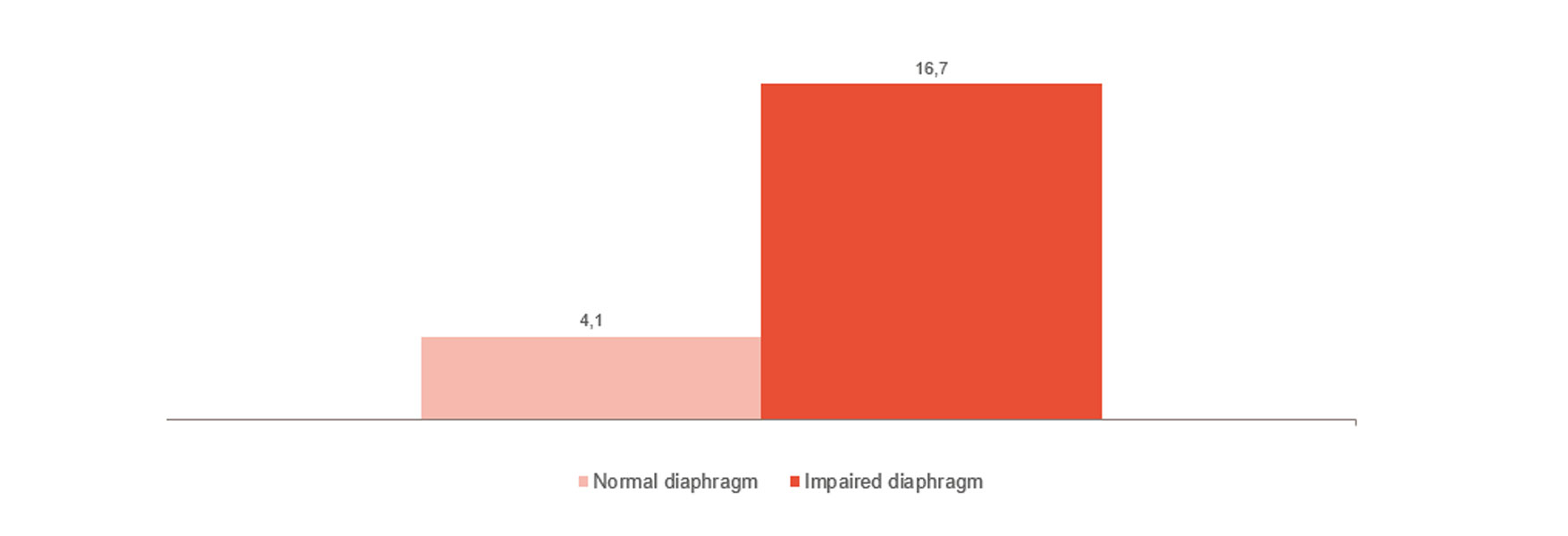
Monitor and trend weaning
As shown in the image, diaphragm dysfunction is strongly linked to weaning difficulties.[4] Monitoring diaphragm activity can help you predict weaning readiness and monitor its progress.[24],[25],[26] All the way from invasive ventilation to non-invasive ventilation, high-flow therapy and when all support has been removed.
The ability of your patient to cope with reduced support is trended within minutes and can help you push-on or fine-tune the support. It may be necessary to go back to your previous settings to prevent your patient from relapsing and the complications which often ensue.
