Advanta V12 balloon expandable covered stent
Advanta V12 is the first to market balloon expandable, fully encapsulated stent that has served physicians with more than 850,000 units sold. Known for its precision and predictability – the versatile Advanta V12 covered stent has been meeting the needs of surgeons and patients for 20 years, and is the only durable solution backed by decades of real-world evidence.[1][2]
Indications:
The Advanta V12 balloon expandable covered stent is indicated for restoring and improving the patency of the iliac and renal arteries. Renal approval includes 5mm, 6mm and 7mm diameter Advanta V12 sizes. In Canada, the Advanta V12 covered stent indication excludes renal arteries.
The Advanta V12 balloon expandable covered stent is not available in the U.S.


Predictable & Precise
-
Designed for secure delivery & placement: Average stent securement force is 2-4 times higher than peak insertion forces [1]
-
6 French compatible with most common renal sizes* [1]
-
Radiopaque markers enhance visibility during deployment and assist with accurate stent placement [1]
-
Designed for pushability and trackability through tortuous anatomy with conformance to iliac and renal arteries [1]
-
Predictable recoil and foreshortening promotes precise deployment [1]
* Please see the Advanta V12 order information found in the documents tab for detailed compatibility

Versatile & Flexible
- Low profile, reliable stent retention force, and secure trackability facilitate the stent implantation [1]
-
Full encapsulation with ePTFE minimizes neointimal hyperplasia formation and helps mitigate the risks related to vessel perforation [1][7][10]
-
Smooth inner lumen offers ease of navigation during re-intervention [1]
-
Able to post-dilate and flare stent: conforming to the anatomy and customizing each patient's treatment [1][9]
-
316L stainless steel struts provide additional radial force, designed to support the patency of the stent [1]
-
Dog-bone inflation design is intended to reduce the chances of embolization[8]
COBEST - 5 year results: Advanta V12 vs. Bare Metal Stent [3]
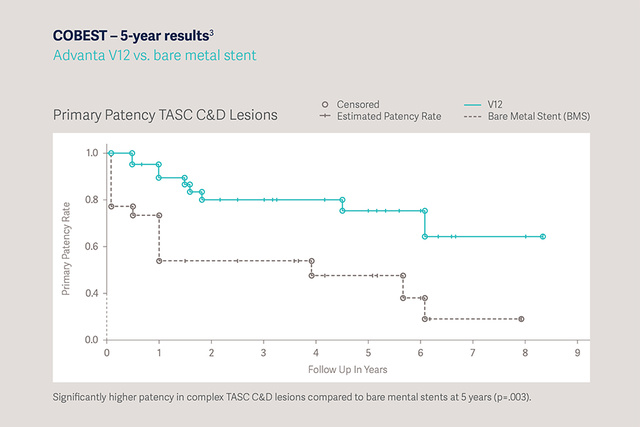
- Significantly higher patency in complex TASC C&D lesions compared to bare metal stents at 5 years (p=0.003).
- Proven two-fold lower reintervention compared to bare metal stents at 5 years post-procedure.
Systematic review of covered balloon-expandable stents for treating aortoiliac occlusive disease [2]
- The Advanta V12/iCast balloon-expandable covered stent has long-term, real-world follow-up, including a reported 5-year primary patency rate of 74.7%.
- The Advanta V12/iCast was the most common device studied in the literature from 2000-2019 (10/15 publications; 66.7%).
- The Advanta V12/iCast studies treated patients with more severe disease (a greater number of TASC C&D lesions) and more severe symptoms (more Rutherford classification 4 & 5) compared to patients enrolled in clinical trials studying other covered balloon-expandable stents.
- Freedom from TLR: Results were comparable for all covered balloon-expandable stents at 1 year. The Advanta V12/iCast has long-term target lesion revascularization data.

iCARUS: Single-Arm IDE study with 3-year follow-up [5]
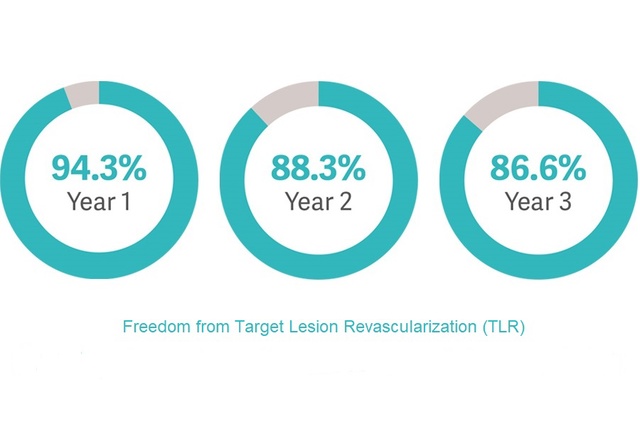
- Real-world patient population with multiple lesions and bilateral disease.
- Study showed sustained clinical benefit with freedom from Target Lesion Revascularization (TLR) up to 3 years.
Pre and post images from occlusive disease treatment with Advanta V12
Bilateral iliac artery occlusion
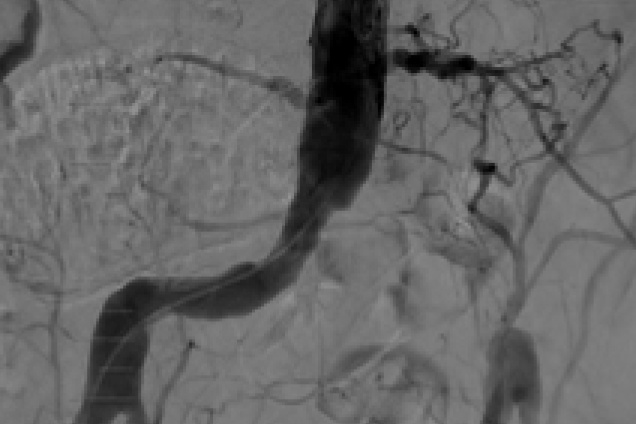
Pre treatment
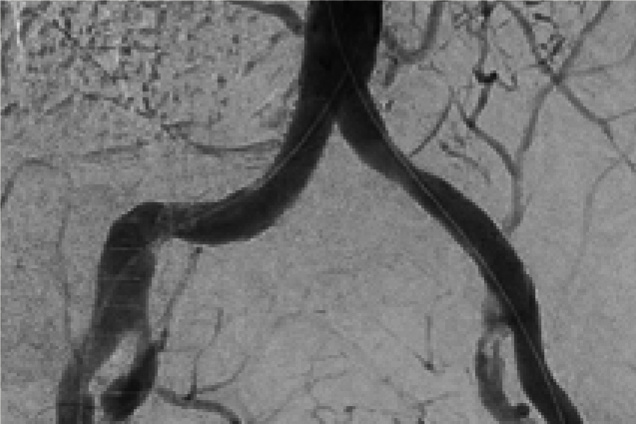
Post treatment
Restoration of the lumen diameter with 10x38 mm Advanta V12 covered stent in RIA; 10x59 mm and 10x38 mm Advanta V12 covered stents overlapped in LIA.
Bilateral common iliac artery occlusion
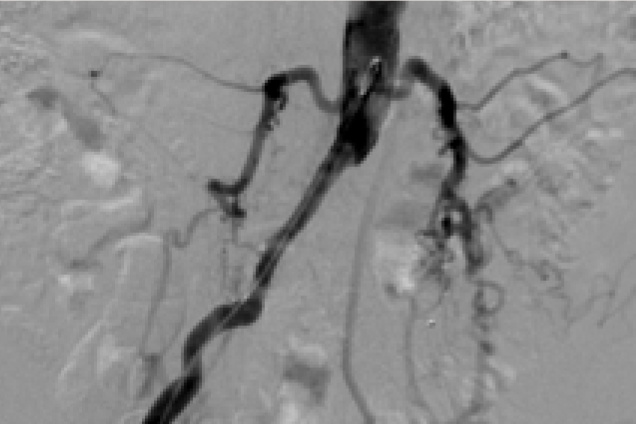
Pre treatment
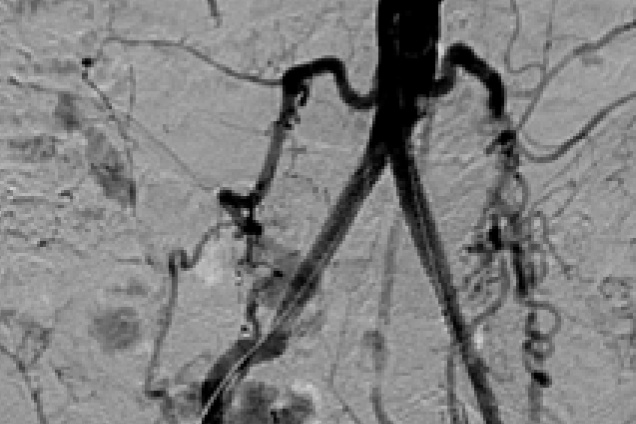
Post treatment
Restoration of the lumen diameter with 8x59 mm Advanta V12 covered stents in RIA and LIA.
Renal artery stenosis
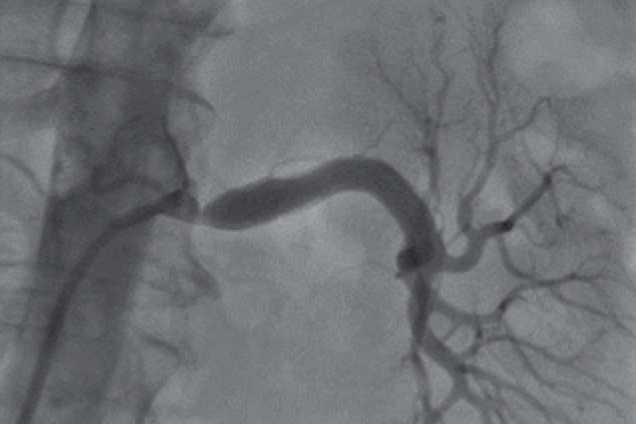
Pre treatment
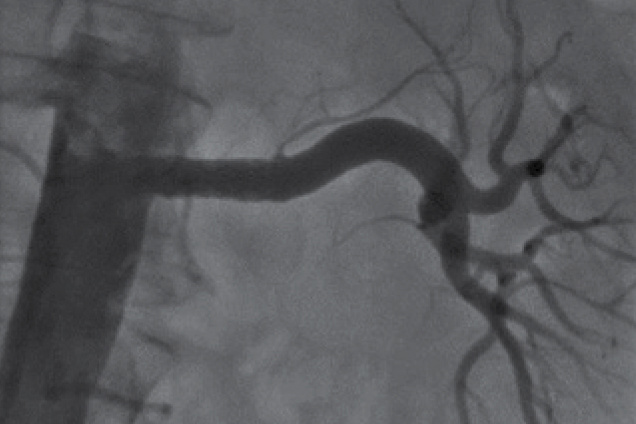
Post treatment
Restoration of the lumen diameter with Advanta V12 covered stents in LRA.
RIA - Right Iliac Artery, LIA - Left Iliac Artery, LRA - Left Renal Artery, RRA - Right Renal Artery
Marketing Sales - Sales Flyer
-
Advanta V12 Ordering Information Sheet