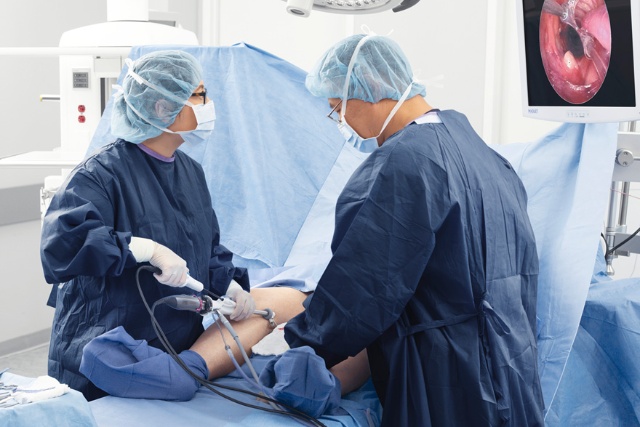
Proven designs and professional support in cardiovascular and cardiothoracic therapy
As your partner for cardiovascular and cardiothoracic procedures, we offer a broad portfolio of cutting edge solutions for Intra-Aortic Balloon Pump Therapy, endoscopic vessel harvesting, and beating heart surgery. Our vascular graft technology is the result of 50 years of technical and clinical experience, and Getinge is also proud to offer the industry-leading Atrium family of thoracic drains.
Explore our products
Find the right products and solutions for you